Hep B biotech Antios closed after FDA hold proved insurmountable
Por um escritor misterioso
Descrição
Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable. | Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable.

Landon Loving on LinkedIn: Selecta, Sobi rout gout in pair of

IHEP (International Hepatology Education Program)

Landon Loving sur LinkedIn : GSK's $100M ADC bet in doubt after

Hepatitis B Foundation
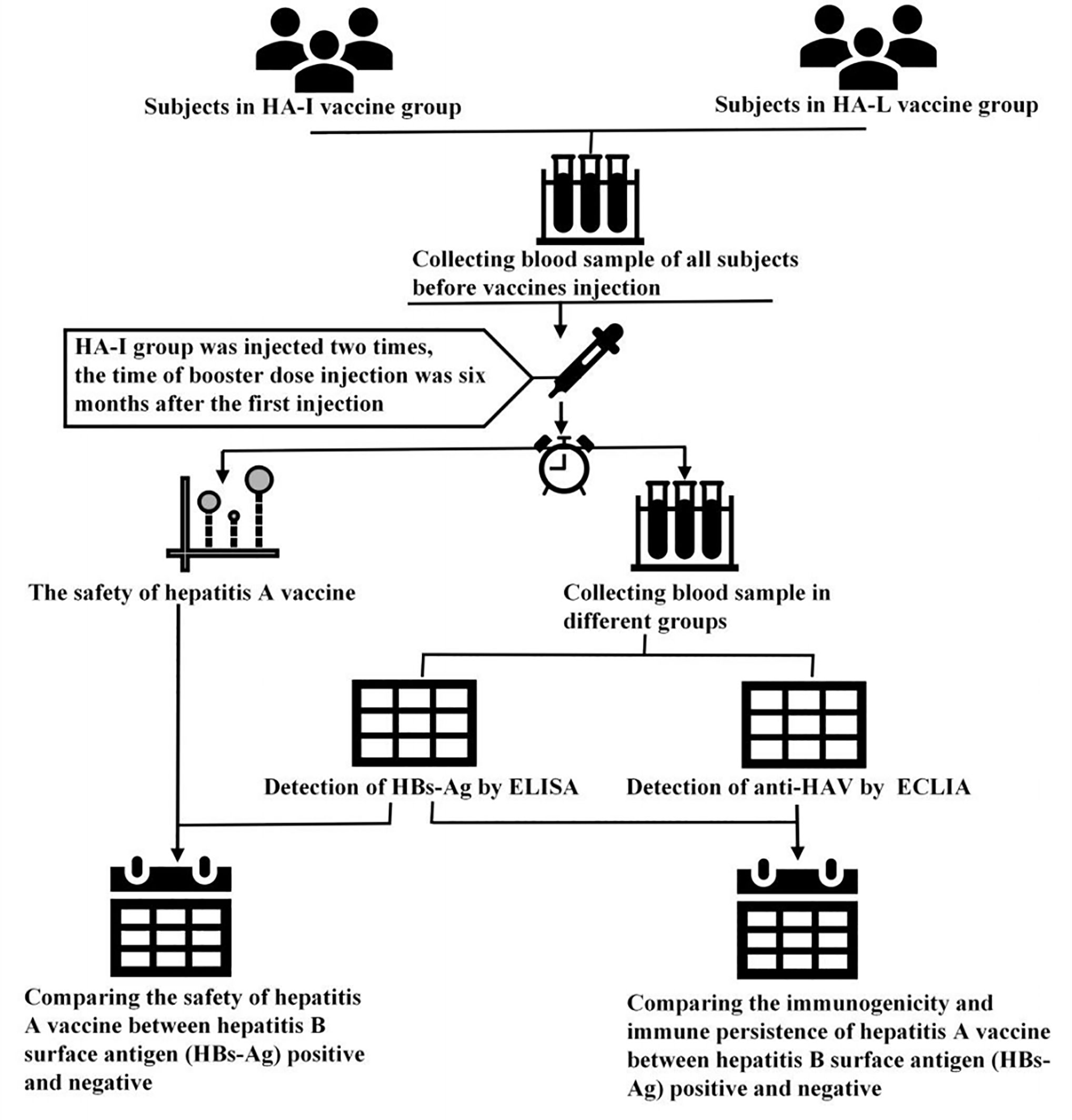
Frontiers The Safety, Immunogenicity, and Immunopersistence of
Annalee Armstrong - Journalist Profile - Intelligent Relations

Former J&J R&D chief Mathai Mammen lands at FogPharma
Biotech Fierce Biotech

Antios rocked as hepatitis B safety signal sparks clinical hold
de
por adulto (o preço varia de acordo com o tamanho do grupo)